Dmitri Mendeleev was able to create a unique table of chemical elements, the main advantage of which was periodicity. Metals and non-metals in the periodic table are arranged in such a way that their properties change in a periodic manner.
The periodic system was compiled by Dmitri Mendeleev in the second half of the 19th century. The discovery not only made it possible to simplify the work of chemists, she was able to combine all discovered chemicals in herself as a single system, as well as predict future discoveries.
The creation of this structured system is priceless for science and for humanity as a whole. It was this discovery that gave impetus to the development of all chemistry for many years.
Interesting to know! There is a legend that the scientist saw the finished system in a dream.
In an interview with one journalist, the scientist explained that he had been working on it for 25 years and that he dreamed about it was quite natural, but this does not mean that all the answers came in a dream.
The system created by Mendeleev is divided into two parts:
- periods - horizontal columns in one or two lines (rows);
- groups - vertical lines, in one row.

In total, there are 7 periods in the system, each next element differs from the previous one by a large number of electrons in the nucleus, i.e. the charge of the nucleus of each right indicator is greater than the left one by one. Each period begins with a metal, and ends with an inert gas - this is precisely the periodicity of the table, because the properties of compounds change within one period and repeat in the next. At the same time, it should be remembered that periods 1-3 are incomplete or small, they have only 2, 8 and 8 representatives. In the full period (i.e. the remaining four) 18 chemical representatives.
 The group contains chemical compounds with the same highest, i.e. they have the same electronic structure. A total of 18 groups are represented in the system ( full version), each of which begins with alkali and ends with an inert gas. All substances presented in the system can be divided into two main groups - metal or non-metal.
The group contains chemical compounds with the same highest, i.e. they have the same electronic structure. A total of 18 groups are represented in the system ( full version), each of which begins with alkali and ends with an inert gas. All substances presented in the system can be divided into two main groups - metal or non-metal.
To facilitate the search, the groups have their own name, and the metallic properties of the substances increase with each lower line, i.e. the lower the compound, the more atomic orbits it will have and the weaker the electronic bonds. The crystal lattice also changes - it becomes pronounced in elements with a large number of atomic orbits.
In chemistry, three types of tables are used:
- Short - actinides and lanthanides are taken out of the boundaries of the main field, and 4 and all subsequent periods occupy 2 lines each.
- Long - in it actinides and lanthanides are taken out of the boundary of the main field.
- Extra long - each period occupies exactly 1 line.
The main one is considered to be the periodic table, which was adopted and officially confirmed, but for convenience, the short version is more often used. Metals and non-metals in the periodic table are arranged according to strict rules that make it easier to work with it.
Metals in the periodic table
 In the Mendeleev system, alloys have a predominant number and their list is very large - they start with Boron (B) and end with polonium (Po) (the exceptions are germanium (Ge) and antimony (Sb)). This group has characteristic features, they are divided into groups, but their properties are heterogeneous. Their characteristic features:
In the Mendeleev system, alloys have a predominant number and their list is very large - they start with Boron (B) and end with polonium (Po) (the exceptions are germanium (Ge) and antimony (Sb)). This group has characteristic features, they are divided into groups, but their properties are heterogeneous. Their characteristic features:
- plastic;
- electrical conductivity;
- shine;
- easy return of electrons;
- ductility;
- thermal conductivity;
- hardness (except mercury).
Due to the different chemical and physical essence, the properties can differ significantly between two representatives of this group, not all of them are similar to typical natural alloys, for example, mercury is a liquid substance, but belongs to this group.
 In its normal state, it is liquid and without crystal lattice, which plays a key role in alloys. Only chemical characteristics make mercury related to this group of elements, despite the conditionality of the properties of these organic compounds. The same applies to cesium - the softest alloy, but it cannot exist in nature in pure.
In its normal state, it is liquid and without crystal lattice, which plays a key role in alloys. Only chemical characteristics make mercury related to this group of elements, despite the conditionality of the properties of these organic compounds. The same applies to cesium - the softest alloy, but it cannot exist in nature in pure.
Some elements of this type can exist only for a fraction of a second, and some do not occur in nature at all - they were created in artificial laboratory conditions. Each of the metal groups in the system has its own name and features that distinguish them from other groups.
However, their differences are quite significant. In the periodic system, all metals are arranged according to the number of electrons in the nucleus, i.e. by increase atomic mass. At the same time, they are characterized by a periodic change in their characteristic properties. Because of this, they are not placed neatly in the table, but may be incorrect.
 In the first group of alkalis, there are no substances that would be found in pure form in nature - they can only be in the composition of various compounds.
In the first group of alkalis, there are no substances that would be found in pure form in nature - they can only be in the composition of various compounds.
How to distinguish metal from non-metal?
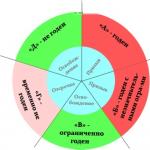
How to determine the metal in the compound? There is an easy way to determine, but for this you need to have a ruler and a periodic table. To determine you need:
- Draw a conditional line along the junctions of elements from Bor to Polonium (possible to Astatine).
- All materials that will be on the left of the line and in the side subgroups are metal.
- The substances on the right are of a different type.
However, the method has a flaw - it does not include germanium and antimony in the group and works only in a long table. The method can be used as a cheat sheet, but in order to accurately determine the substance, you should remember a list of all non-metals. How many are there? Few - only 22 substances.
 In any case, to determine the nature of a substance, it is necessary to consider it separately. The elements will be easy if you know their properties. It is important to remember that all metals:
In any case, to determine the nature of a substance, it is necessary to consider it separately. The elements will be easy if you know their properties. It is important to remember that all metals:
- At room temperature they are solid except for mercury. At the same time, they shine and conduct well. electricity.
- They have a smaller number of atoms at the outer level of the nucleus.
- Consist of a crystal lattice (except mercury), and all other elements have a molecular or ionic structure.
- In the periodic table, all non-metals are red, metals are black and green.
- If you move from left to right in a period, then the charge of the nucleus of matter will increase.
- Some substances have weak properties, but they still have characteristic features. Such elements belong to semimetals, such as Polonium or Antimony, they are usually located on the border of two groups.
Attention! In the lower left part of the block in the system there are always typical metals, and in the upper right - typical gases and liquids.
It is important to remember that when moving from top to bottom in the table, the non-metallic properties of substances become stronger, since there are elements that have distant outer shells. Their nucleus is separated from the electrons and therefore they are attracted weaker.
Useful video
Summing up
It will be easy to distinguish elements if you know the basic principles for the formation of the periodic table and the properties of metals. It will also be useful to memorize the list of the remaining 22 elements. But we must not forget that any element in the compound should be considered separately, not taking into account its bonds with other substances.
In contact with
Non-metals - elements that have non-metallic properties and occupy a position in the upper right corner in the periodic table. What is the nature of non-metals, as well as how they differ from other compounds, we learn in this article.
general characteristics
Non-metallic elements include p-elements, as well as hydrogen and helium, which in turn belong to s-elements. They are located to the right and above the boron-astatine diagonal. In total, 22 non-metals are known. In the most typical non-metals, the filling of the outer level with electrons is close to the maximum, and the atomic radii are minimal among the elements of this period.

Rice. 1. The group of non-metals in the periodic system.
Atoms of non-metals have higher electronegativity values, and, accordingly, high ionization energies and high electron affinity. In this regard, the nature of non-metals is such that, unlike metals, they can exhibit oxidizing properties. In reactions, they can be restored by adding so many electrons that their total number at the outer level reaches eight (the completed level, the stable state of the atom).
That is why the negative value of the oxidation state that non-metals can have in compounds, unlike metals, is equal to the difference (8-N groups). Non-metals have the highest electronegativity, the position of which falls on the upper right corner in the Periodic Table, that is, the halogens fluorine and chlorine, as well as oxygen. These elements can form ionic bonds. The most active non-metal is fluorine, which in compounds can exhibit only one valence I and one oxidation state -1.
The structural features of non-metals are that the outer electron layer of most non-metal atoms contains from 4 to 8 electrons.
Other non-metals (except fluorine) can also exhibit positive oxidation states, forming covalent bonds with other elements.
Physical properties
For most non-metals of simple substances in the solid state of aggregation, a molecular crystal lattice is characteristic. That is, these non-metals are crystalline substances. Therefore, under normal conditions, they have the form of gases, liquids or solids with low temperatures melting. Examples of such substances are gases: hydrogen H 2, neon Ne, liquid - bromine Br 2, solids iodine I 2, sulfur S 8, phosphorus P 4 (white phosphorus). There are non-metals (boron, carbon, silicon) that have atomic crystal lattices.

Rice. 2. Non-metals - liquids, gases, solids.
The most important elements that are contained in living organisms are organogens. They form water, proteins, vitamins, fats. These include 6 elements: carbon, oxygen, hydrogen, nitrogen, phosphorus, sulfur.
Chemical properties and compounds
Hydrogen compounds of non-metals are mainly volatile compounds, in aqueous solutions having an acidic character. They have molecular structures, a covalent polar bond. Some of them (water, ammonia, hydrogen fluoride) form hydrogen bonds. Compounds are formed by the direct interaction of non-metals with hydrogen. The electronic formula of sulfur with hydrogen is as follows:
S + H 2 \u003d H 2 S (up to 350 degrees, the balance is shifted to the right)
All hydrogen compounds are reducing agents (except HF), and their reducing power increases from right to left along the period and from top to bottom in the subgroup.
Nonmetals interact with metals and other nonmetals:
The result is the sodium salt of hydrochloric acid.

Rice. 3. sodium salt of hydrochloric acid.
compounds of non-metals with oxygen, as a rule, are acidic oxides, which correspond to oxygen-containing acids. The structure of oxides of typical non-metals is molecular (SO 3, P 4 O 10). The higher the oxidation state of the nonmetal, the stronger the corresponding oxoacid. So, chlorine does not directly interact with oxygen, but forms a number of oxo acids, which correspond to oxides, anhydrides of these acids.
Non-metals are used in various industries. Here is a list of industries where their use is most in demand.
| Application area | Examples, a list of non-metals used in a particular industry |
| industry | Sulfur, nitrogen and phosphorus are often used to produce acids. Sulfur is also used in the production of rubber. |
| transport | Hydrogen is an important non-metal in the transport industry. It is used as a fuel. When burned, this type of fuel does not pollute the environment. |
| agricultural industry | sulfur is used to control harmful insects and plant diseases |
| the medicine | Oxygen is used to restore breathing (oxygen pillows), coal in the form activated carbon which is able to remove harmful substances from the body. |
| food industry | nitrogen is used to extend the shelf life of products |
What have we learned?
This article for grade 9 chemistry summarizes the basic information about non-metals, their structure, and what non-metals react with. Non-metals can be gases, liquids and solids with a crystal lattice. The most reactive non-metal is fluorine, which has an oxidation state of -1.
Topic quiz
Report Evaluation
Average rating: 4.1. Total ratings received: 352.
- this is the ability to polarize a chemical bond, to pull common electron pairs towards itself.
22 elements are classified as non-metals.
The position of non-metallic elements in the periodic table of chemical elements
| Group | I | III | IV | V | VI | VII | VIII |
| 1st period | H | He | |||||
| 2nd period | AT | FROM | N | O | F | Ne | |
| 3rd period | Si | P | S | CL | Ar | ||
| 4th period | As | Se | Br | kr | |||
| 5th period | Te | I | Xe | ||||
| 6th period | At | Rn |
The structure of atoms of non-metals
A characteristic feature of non-metals is a larger (compared with metals) number of electrons at the external energy level of their atoms. This determines their greater ability to add additional electrons and exhibit higher oxidative activity than metals. Particularly strong oxidizing properties, i.e., the ability to attach electrons, are exhibited by non-metals that are in the 2nd and 3rd periods of groups VI-VII. If we compare the arrangement of electrons in orbitals in the atoms of fluorine, chlorine and other halogens, then we can also judge their distinctive properties. The fluorine atom has no free orbitals. Therefore, fluorine atoms can only show valency I and oxidation state - 1. The strongest oxidizing agent is fluorine. In the atoms of other halogens, for example, in the chlorine atom, there are free d-orbitals at the same energy level. Due to this, the depairing of electrons can occur in three different ways. In the first case, chlorine can exhibit an oxidation state of +3 and form hydrochloric acid HClO 2 , which corresponds to salts - chlorites, for example, potassium chlorite KClO 2 . In the second case, chlorine can form compounds in which the oxidation state of chlorine is +5. Such compounds include chloric acid HClO 3 and its salts, chlorates, for example, potassium chlorate KClO 3 (Bertolet's salt). In the third case, chlorine exhibits an oxidation state of +7, for example, in perchloric acid HClO 4 and in its salts, perchlorates (in potassium perchlorate KClO 4).Structures of non-metal molecules. Physical properties of non-metals
In the gaseous state at room temperature are:· hydrogen - H 2 ;
· nitrogen, N 2 ;
· oxygen - O 2 ;
· fluorine - F 2 ;
· chlorine - CI 2 .
And inert gases:· helium - He;
· neon - Ne;
· argon - Ar;
· krypton, Kr;
· xenon - Xe;
· radon - Rn).
AT liquid- bromine - Br.AT solid:
Tellurium - Te;
· iodine - I;
· astatine - At.
Non-metals also have a much richer spectrum of colors: red for phosphorus, brown for bromine, yellow for sulfur, yellow-green for chlorine, purple for iodine vapor, etc.The most typical non-metals have a molecular structure, while the less typical ones have a non-molecular structure. This explains the difference in their properties.
Composition and properties of simple substances - non-metals
Non-metals form both monatomic and diatomic molecules. To monatomic non-metals include inert gases that practically do not react even with the most active substances. Inert gases are located in group VIII of the periodic system, and the chemical formulas of the corresponding simple substances are as follows: He, Ne, Ar, Kr, Xe and Rn.
Some nonmetals form diatomic molecules. These are H 2, F 2, Cl 2, Br 2, Cl 2 (elements of group VII of the periodic system), as well as oxygen O 2 and nitrogen N 2. From triatomic molecules consists of ozone gas (O 3). For substances of non-metals that are in the solid state, it is quite difficult to make a chemical formula. The carbon atoms in graphite are connected to each other in various ways. It is difficult to isolate an individual molecule in the given structures. When writing the chemical formulas of such substances, as in the case of metals, the assumption is introduced that such substances consist only of atoms. At the same time, chemical formulas are written without indices: C, Si, S, etc. Such simple substances as ozone and oxygen, which have the same qualitative composition (both consist of the same element - oxygen), but differ in number atoms in a molecule have different properties. So, oxygen has no smell, while ozone has a pungent smell that we feel during a thunderstorm. The properties of solid non-metals, graphite and diamond, which also have the same qualitative composition but different structure, differ sharply (graphite is brittle, diamond is hard). Thus, the properties of a substance are determined not only by its qualitative composition, but also by how many atoms are contained in a substance molecule and how they are interconnected. Non-metals in the form of simple bodies are in a solid or gaseous state (excluding bromine - liquid). They do not have the physical properties of metals. Solid non-metals do not have the characteristic brilliance of metals, they are usually brittle, poorly conduct electricity and heat (with the exception of graphite). Crystalline boron B (like crystalline silicon) has a very high melting point (2075°C) and high hardness. The electrical conductivity of boron increases greatly with increasing temperature, which makes it possible to widely use it in semiconductor technology. The addition of boron to steel and alloys of aluminum, copper, nickel, etc. improves their mechanical properties. Borides (compounds of boron with some metals, for example, with titanium: TiB, TiB 2) are necessary in the manufacture of jet engine parts, gas turbine blades. As can be seen from Scheme 1, carbon is C, silicon is Si, and boron is B have a similar structure and have some common properties. As simple substances, they occur in two modifications - crystalline and amorphous. The crystalline modifications of these elements are very hard, with high melting points. Crystalline silicon has semiconductor properties. All these elements form compounds with metals - carbides, silicides and borides (CaC 2 , Al 4 C 3 , Fe 3 C, Mg 2 Si, TiB, TiB 2). Some of them have greater hardness, such as Fe 3 C, TiB. Calcium carbide is used to produce acetylene.
Non-metals are elements that differ significantly in physical and chemical properties from metals. The reason for their differences could be explained in detail only at the end of the 19th century, after the discovery of the electronic structure of the atom. What is the peculiarity of non-metals? What qualities are characteristic of their day? Let's figure it out.
Non-metals - what is it?
The approach to separating elements into metals and non-metals has long existed in the scientific community. The first elements in the periodic table of Mendeleev usually include 94 elements. Mendeleev's non-metals include 22 elements. In they occupy the upper right corner.
In their free form, non-metals are simple substances, main feature which is the absence of characteristic metallic properties. They can be in all states of aggregation. So, iodine, phosphorus, sulfur, carbon are found in the form of solid substances. The gaseous state is characteristic of oxygen, nitrogen, fluorine, etc. Only bromine is a liquid.
In nature, non-metal elements can exist both in the form of simple substances and in the form of compounds. Sulfur, nitrogen, oxygen are found in unbound form. In compounds, they form borates, phosphates, etc. In this form, they are present in minerals, water, rocks Oh.
Difference from metals
Non-metals are elements that are different from metals appearance, structure and chemical properties. They have a large number of unpaired electrons at the outer level, which means they are more active in oxidative reactions and easier to attach additional electrons to themselves.
A characteristic difference between the elements is observed in the structure of the crystal lattice. In metals, it is metallic. In non-metals, it can be of two types: atomic and molecular. The atomic lattice gives substances hardness and increases the melting point; it is characteristic of silicon, boron, and germanium. Chlorine, sulfur, oxygen have a molecular lattice. It gives them volatility and a little hardness.
The internal structure of the elements determines them physical properties. Metals have a characteristic luster, good conductivity of current and heat. They are hard, ductile, malleable, have a small range of colors (black, shades of gray, sometimes yellowish).
Non-metals are liquid, gaseous or non-lustrous and malleable. Their colors vary greatly and can be red, black, gray, yellow, etc. Almost all non-metals are poor conductors of current (except carbon) and heat (except black phosphorus and carbon).

Chemical properties of non-metals
AT chemical reactions non-metals can act as both oxidizing and reducing agents. When interacting with metals, they take on electrons, thus exhibiting oxidizing properties.
Interacting with other non-metals, they behave differently. In such reactions, the less electronegative element acts as a reducing agent, while the more electronegative element acts as an oxidizing agent.
With oxygen, almost all (except fluorine) non-metals act as reducing agents. When interacting with hydrogen, many are oxidizing agents, subsequently forming volatile compounds.
Some non-metal elements have the ability to form several simple substances or modifications. This phenomenon is called allotropy. For example, carbon exists in the form of graphite, diamond, carbine, and other modifications. Oxygen has two of them - ozone and oxygen itself. Phosphorus comes in red, black, white, and metallic.

Nonmetals in nature
Non-metals are found everywhere in varying amounts. They are part of the earth's crust, are part of the atmosphere, hydrosphere, are present in the Universe and in living organisms. In outer space, the most common are hydrogen and helium.
On Earth, the situation is quite different. The most important constituents of the earth's crust are oxygen and silicon. They make up more than 75% of its mass. But the smallest amount falls on iodine and bromine.
In the composition of sea water, oxygen accounts for 85.80%, and hydrogen - 10.67%. Its composition also includes chlorine, sulfur, boron, bromine, carbon, fluorine and silicon. Nitrogen (78%) and oxygen (21%) dominate in the composition of the atmosphere.

Non-metals such as carbon, hydrogen, phosphorus, sulfur, oxygen and nitrogen are important organic matter. They support the vital activity of all living beings on our planet, including humans.