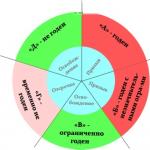
Test on the topic "Classification of chemical reactions"
Specify the reaction schemes of the compound:
1) Fe(OH) 3 → Fe 2 O 3 + H 2 O;
2) AgNO 3 → Ag + NO 2 + O 2;
3) SO 2 + O 2 → SO 3;
4) NO 2 + O 2 + H 2 O → HNO 3.
Specify the reaction schemes for which a catalyst is necessarily used:
1) KOH + H 2 SO 4 → K 2 SO 4 + H 2 O;
2) N 2 + H 2 → NH 3;
3) NH 3 + O 2 → NO + H 2 O;
4) NH 3 + O 2 → N 2 + H 2 O.
Mark the equations or decomposition reaction schemes:
1) HNO 3 → H 2 O + NO 2 + O 2;
2) Fe + Cu(NO 3) 2 = Fe(NO 3) 2 + Cu;
3) NaOH + HCl = NaCl + H 2 O;
4) H 2 O 2 → H 2 O + O 2.
Specify equations or schemes of substitution reactions:
1) Zn + 2AgNO 3 = Zn(NO 3) 2 + 2Ag;
2) Fe + H 2 SO 4 = FeSO 4 + H 2;
3) CaCl 2 + Na 2 CO 3 = CaCO 3 + 2NaCl;
4) CuO + H 2 → Cu + H 2 O.
Mark the equations or schemes of exothermic reactions:
1) N 2 + H 2 → NH 3 + Q;
2) SO 2 + O 2 + Q→ SO 3 ;
3) C + O 2 = CO 2 + 369 kJ;
4) CaCO 3 \u003d CaO + CO 2 + 157 kJ.
Specify equations or schemes of exchange reactions:
1) NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O;
2) Ba(NO 3) 2 + K 2 SO 4 → BaSO 4 + KNO 3;
3) N 2 O 5 + H 2 O → HNO 3;
4) CuO + 2HNO 3 = Cu(NO 3) 2 + H 2 O.
The number of products is always less than the number of starting substances in the case of reactions:
1) exchange;
2) connections;
3) decomposition;
4) substitutions.
Mark the equations or schemes of redox reactions:
1) Al + Cl 2 → AlCl 3;
2) BaCO 3 \u003d BaO + CO 2;
3) H 2 O 2 → H 2 O + O 2;
4) Cu + AgNO 3 → Cu(NO 3) 2 + Ag.
Specify equations or schemes of irreversible reactions:
1) N 2 + 3H 2 ⇆ 2NH 3;
2) Ba(OH) 2 + H 2 SO 4 → BaSO 4 + H 2 O;
3) NaOH + HCl = NaCl + H 2 O;
4) SO 2 + O 2 ⇆ SO 3.
The number of products is always greater than the number of starting substances in the case of reactions:
1) expansions;
2) exchange;
3) connections;
4) substitutions.
redox in inorganic chemistry always the reaction is:
1) expansions;
2) exchange;
3) substitution;
4) connections.
In inorganic chemistry, the substitution reaction proceeds:
1) between two simple substances;
2) two complex substances;
3) simple and complex substances;
4) both two simple and two complex substances.
Redox reactions can be:
1) substitution;
2) exchange;
3) decomposition;
4) connections.
Without changing the oxidation state of the atoms of the elements, reactions always proceed:
1) exchange;
2) connections;
3) decomposition;
4) substitutions.
Substances can enter into an exchange reaction with each other:
1) simple and complex;
2) two simple ones;
3) two complex;
4) both two simple ones and two complex ones.
As a result of the decomposition reaction, the following can be formed:
1) only simple substances;
2) only complex substances;
3) either only simple or only complex substances;
4) both simple and complex substances.
Substances can react with each other:
1) only simple;
2) only complex;
3) both simple and complex;
4) simple with complex.
Specify the correct statements:
1) as a result of the decomposition reaction, no more than two new substances are formed;
2) in an exchange reaction, the number of products with different compositions is always equal to the number of starting substances with different compositions;
3) in a compound reaction, the number of starting substances of different composition is always greater than the number of products;
4) in inorganic substitution reactions, the number of starting substances with different composition is equal to the number of products with different composition.
Indicate the scheme or equation of the redox decomposition reaction:
1) CaCO 3 \u003d CaO + CO 2;
2) H 2 O 2 → H 2 O + O 2;
3) Cu + O 2 → CuO;
4) NaHCO 3 → Na 2 CO 3 + H 2 O + CO 2.
Mark the equations or schemes of the redox reactions of the compound:
1) SO 2 + O 2 → SO 3;
2) NO 2 + H 2 O → HNO 3 + NO;
3) NO 2 + O 2 + H 2 O → HNO 3;
4) SO 2 + NaOH \u003d NaHSO 3.
Specify schemes or equations of redox substitution reactions:
1) CaCO 3 + SiO 2 = CaSiO 3 + CO 2;
2) Fe(OH) 2 + HCl → FeCl 2 + H 2 O;
3) Zn + H 2 SO 4 \u003d ZnSO 4 + H 2;
4) Fe 2 O 3 + H 2 → Fe + H 2 O.
ReactionNOTcan be at the same time:
1) exchange and redox;
2) redox and decomposition;
3) substitution and redox;
4) compounds and redox.
Homogeneous can be:
1) only exchange reactions;
2) only compound reactions;
3) only decomposition reactions;
4) all the above reactions, as well as substitution reactions.
The same reaction can be simultaneously:
a) reversible and exothermic;
b) substitution and exchange;
c) exchange and decomposition;
d) compounds and decompositions.
Mark equations or schemes of homogeneous compound reactions:
1) CaO (tv) + CO 2 (g) = CaCO 3 (tv);
2) CO (g) + O 2 (g) → CO 2 (g);
3) NO 2 (g) + H 2 O (g) + O 2 (g) → HNO 3 (g);
4) SO 3 (l) + H 2 O (l) \u003d H 2 SO 4 (l).
Specify the equations or schemes of homogeneous redox decomposition reactions:
1) Cu(NO 3) 2 (tv) → CuO (tv) + NO 2 (g) + O 2 (g);
2) SO 3 (g) → SO 2 (g) + O 2 (g);
3) NH 4 NO 3 (tv) → N 2 O (g) + H 2 O (l);
4) 2NH 3 (g) = N 2 (g) + 3H 2 (g).
Mark the equations or schemes of heterogeneous redox reactions:
1) Na (tv) + H 2 O (g) → NaOH (p-p) + H 2 (g);
2) H 2 (g) + CuO (tv) = Cu (tv) + H 2 O (g);
3) NO 2 (g) + CO (g) → N 2 (g) + CO 2 (g);
4) H 2 (g) + N 2 O (g) \u003d N 2 (g) + H 2 O (g).
Specify schemes or equations of homogeneous exothermic exchange reactions:
1) NaOH (p-p) + HCl (p-p) = NaOH (p-p) + H 2 O (g) + Q;
2) Ba(OH) 2 (p-p) + 2HCl (p-p) = BaCl 2 (p-p) + 2H 2 O (g) + Q;
3) N 2 (g) + H 2 (g) → NH 3 (g) + Q;
4) H 2 (g) + I 2 (g) → HI (g) − Q.
Schemes (equations) of reactions for obtaining SO are given 2 . Of these, heterogeneous redox reactions will be:
a) FeS 2 (tv) + O 2 (g) → Fe 2 O 3 (tv) + SO 2 (g);
b) NaHSO 3 (p-p) + HCl (p-p) → NaCl (p-p) + H 2 O (g) + SO 2 (g);
c) S (tv) + O 2 (g) \u003d SO 2 (g);
d) Fe 2 (SO 4) 3 (tv) → Fe 2 O 3 (tv) + SO 2 (g) + O 2 (g).
Reactions are given: a) firing of pyrite; b) limestone calcination; c) photosynthesis; d) interaction of aqueous solutions of sodium sulfate and barium chloride. Of the following, redox reactions will be:
The following signs are suitable for describing the neutralization reaction:
1) always homogeneous;
2) exchange;
3) products - usually salt and water;
4) can be homogeneous and heterogeneous.
Substances are given whose formulas are Zn, ZnO, Zn(OH) 2 , ZnCO 3 , SO 2 . Specify the number of substances that can react with H 2 SO 4 (razb.) according to the type of exchange reaction:
Substances are given whose formulas are Al 2 O 3 , Al, Al(OH) 3 , Na 3 , Al(NO 3 ) 3 , SO 2 , P 2 O 5 . The number of substances with which KOH (solution) can react according to the type of compound reaction is:
Indicate the formula of the substance with which CuSO 4 (solution) reacts according to the type of exchange reaction:
Substances with formulas Fe are given 2 O 3 , NaHS, Fe(OH) 3 , Ba(NO 3 ) 2 , NaHSO 4 , Na 2 . The number of substances with which H 2 SO 4 (razb.) reacts according to the type of exchange reaction, equal to:
The reactions of the compound should include the reactions:
1) between K 2 S (solution) and H 2 S;
2) Al(OH) 3 and NaOH (p-p);
3) K 3 and HCl (p-p);
4) Al 2 O 3 and KOH (tv).
Specify exothermic processes:
1) F 0 (d) + e - → F − (r);
2) H 2 (g) → 2H (g);
3) Mg (g) → Mg 2+ (g) + 2 e - ;
4) Ca 2+ (g) + 2 e - = Ca(g).
1) The exchange reaction is
a) BaO + HOH →; b) H2SO4 + Zn →; c) HNO3 + Ca(OH)2 →; d) N2 + O2 →
2) The reversible reaction is
a) HCl + KOH → KCl - + H2O; b) N2 + O2 → 2NO;
c) C(s) + O2 → CO2; d) BaCl2 + 2AgNO3 → 2AgCl + Ba(NO3)2
3) Endothermic reactions are:
a) CaCO3 \u003d CaO + CO2 -Q; b) 2H2O2 → 2H3O + O2 + Q
c) N2 + O2 → 2NO -Q; d) 2SO2 + O2 → 2SO3 + Q
4) Catalyst is...
a) a reaction retarder that reacts with the starting materials;
b) a reaction accelerator that reacts with the starting materials;
c) a reaction accelerator that is not consumed and is not included in the products;
5) A homogeneous reaction is:
a) 2H2O2(l) ↔ 2H2O(l) + O2(g); b) CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(steam);
c) 2Al(s) + 3Cl2(g) → 2AlCl3(s); d) Ca(tv) + 2HCl(l) → CaCl2(l) + H2(g)
6) Not a redox reaction is
a) 2C + O2 → 2CO; b) N2 + O2 → 2NO
c) BaO + SO2 → BaSO3; d) 2Al + 6HCl → 2AlCl3 + 3H2
7) The decomposition reaction is:
a) 2Mg + O2 → 2MgO; b) (CuOH)2CO3 = 2CuO + CO2 + H2O;
c) 3O2 → 2O3; d) CH₄ + 2O₂ → CO₂ + 2H₂O
8) A reaction that proceeds without changing the composition of substances is
a) N2 + O2 → 2NO; b) P4 ↔ 4P; c) 2C + O2 → 2CO; d) N2 + 3H2 → 2NH3
a) FeCl3 + NaOH →
c) Mg + HNO3 →
10) The displacement of less active metals from their oxides by more active metals is ...
a) oxidation; b) combustion; c) metallothermy; d) metal-plastic.
11) Berthollet's rule says:
a) Every pure substance, regardless of the method of its preparation, always has a constant qualitative and quantitative composition.
b) The mass of the substances that entered into the reaction is equal to the mass of the substances formed as a result of it.
c) The volumes of reacting gases are related to each other and to the volumes of gaseous reaction products formed as small integers equal to stoichiometric coefficients.
d) Reactions between electrolyte solutions proceed to completion if a precipitate, a gas, or a low-dissociating substance is formed.
12) Describe the chemical reaction in all respects:
2H2O2(l) ↔ 2Н3O(l) + O2(g) + Q
Test on the topic "Classification of chemical reactions"
1) Exothermic reactions are:
a) Mg + 1/2O2 → MgO +614 kJ; b) H2 + O2 → 2H2O -484 kJ;
c) d) CH₄ + 2O₂ → CO₂ + 2H₂O +891 kJ; d) 2C + 2H2 → C2H4 -55 kJ
2) An inhibitor is...
a) a reagent that slows down or stops a chemical reaction;
b) a reagent that speeds up a chemical reaction;
c) a reagent that monitors the presence of by-products in the reaction system;
d) a means of monitoring the progress of a chemical reaction.
3) The substitution reaction is
a) Na2O + HOH →; b) H2SO4 + Al →; c) AgNO3 + CaCl2 →; d) N2 + H2 →
4) An irreversible reaction is:
a) K2CO3 + HCl → KCl -+ CO2 + H2O; b) N2 + O2 → 2NO;
c) SO2 + 1/2O2 → SO3; d) N2 + 3H2 → 2NH3
5) The redox reaction is:
a) CO2 + Na2O → Na2CO3; b) 2Al + 6HCl → 2AlCl3 + 3H2
c) BaCl2 + K2SO4 → BaSO4 + 2KCl; d) 2HNO3 + CaO → Ca(NO3)2 + H2O
6) A heterogeneous reaction is:
a) 2H2(g) + O2(g) ↔ 2H2O (steam); b) CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(steam);
c) Al(s) + 3HCl(l) → AlCl3(l) + 1/2Н2(g) d) 2Al(s) + 3I2(s) → 2AlI3(s)
7) A reaction that proceeds without changing the composition of substances is:
c) 3C2H2 → C6H6; d) S + O2 → SO2
8) The reaction of the connection is:
a) 2CrO + 1/2O2 → Cr2O3; b) (CuOH)2CO3 → 2CuO + CO2 + H2O;
c) P4 → 4P; d) C2H₄ + 3O₂ → 2CO₂ + 2H₂O
9) Add the reaction equations and determine their type (s.r., r.s., r.z., r.o.):
a) Na + HOH →
d) H3PO4 + NaOH→
10) The interaction of strong acids and bases to form salt and water is ...
a) leaching; b) neutralization; c) oxidation; d) acid-base titration.
11) The law of constancy of the composition reads:
a) The volumes of reacting gases are related to each other and to the volumes of gaseous reaction products formed as small integers equal to stoichiometric coefficients.
b) Reactions between electrolyte solutions proceed to completion if a precipitate, a gas, or a low-dissociating substance is formed.
c) Each pure substance, regardless of the method of its preparation, always has a constant qualitative and quantitative composition.
d) The mass of the substances that entered into the reaction is equal to the mass of the substances formed as a result of it.
12) Describe the chemical reaction in all respects:
2SO2(g) + O2(g) ↔ 2SO3(g) + Q
Test № 1
Option 1
Part A Questions .
Part B Questions drawn uplooking for a match , as well as .
each task of part A is estimated at 0.5 points, tasks of part B are graded differently: a completely correct answer - 1 point, out of three answers only 2 are correct - 0.5 points. The task of part C is evaluated differently: from 0.5 to 3 points. After the work is completed, the scores of correctly completed tasks are summed up during the check, thus obtaining the primary score.
8 - 9 5
6 - 7 4
3 - 5 3
0 - 2 2
Part BUT
Hydrochloric acid reacts more rapidly with
1) hg 2) Zn 3) mg 4 )Fe
Reaction does not apply to OVR
1) Al+O 2 → Al 2 O 3
2 ) MnO 2 + H 2 → Mn+H 2 O
3)H 2
O 2
→
H 2
+ O 2
4) HNO 3
+ Fe(OH) 3
→
Fe(NO 3
)
3
+ H 2
O
The reducing agent in the reaction scheme
Mn 2 O 7 + NH 3 → MNO 2 + H 2 O + N 2 is
N 2 0 2) N 3- 3) Mn 4+ 4) Mn 7+
When dissolved in water, hydroxide ions form a substance whose formula is
1)Cu(OH) 2 2)Ca(NO 3 ) 2 3) NaOH 4)H 2 SO 4
5. With complete dissociation, 1 mol of copper nitrate (II) in solution is formed
1) 3 mol of copper cations and 1 mol of nitrate ions
2) 2 mol of copper cations and 3 mol of nitrate ions
3) 1 mol of copper cations and 2 mol of nitrate ions
4) 1 mol of copper cations and 3 mol of nitrate ions
6. Choose the correct notation for the right side of the sodium carbonate dissociation equation
1) = Na + + CO 3 2- 2) = Na + + 2CO 3 2-
3) = 2Na + + CO 3 2- 4) = 2Na + + HCO 3 -
Part B
1) S -2 →S 0 BUT . HNO 3 →H 2 O + NO 2 + O 2
2) S +6 →S +4 B . H 2 S+SO 2 → H 2 O+S
3) N +5 → N +4 AT . NH 3 + O 2 → H 2 O + NO
4) N -3 →N 0 G . NH 3 + O 2 → H 2 O+N 2
D . C+H 2 SO 4 → CO 2 + SO 2 +H 2
1) barium hydroxide and hydrochloric acid A.2H + + SO 3 2- → H 2 O + SO 2
2) iron chloride ( III) and silver nitrate B.Fe 3+ +3 Oh - → Fe(Oh) 3
3) iron nitrate ( III) and potassium hydroxide B.Ag + + Cl - → AgCl
4) sodium sulfite and hydrochloric acid D.H + + Oh - → H 2 O
3 SO 2 + O 2 = 2 SO 3 + Q
VI) necessary
raise the temperature
lower the temperature
reduce pressure
4) increase pressure
5) reduce concentrationO 2
6) increase concentrationSO 2
Part C
To 20 g of a solution containing 5% copper sulfate (II), added sodium hydroxide. Calculate the mass of precipitate formed.
Test work No. 1
on the topic “Classification of chemical reactions. chemical reactions in aqueous solutions"
Option 2
Part A Questions have one correct answer .
Part B Questions drawn uplooking for a match , as well asquestions with multiple answers .
The grading option is: eachthe task of part A is estimated at 0.5 points , taskspart B evaluated differently: completely correct answer - 1 point, out of three answers only 2 are correct - 0.5 points . Exercisepart C assessed differentially: 0.5 to 3 points . After the work is completed, the scores of correctly completed tasks are summed up during the check, thus obtaining the primary score.
Primary score Score in a five-point system
8 - 9 5
6 - 7 4
3 - 5 3
0 - 2 2
Part A
1. The interaction of water with
1) hg 2) Zn 3) mg 4) N a
2 . Reaction does not apply to OVR
1) KMnO 4 → K 2 MNO 4 +MnO 2 + O 2
2) Cu 2 O+ H 2 → Cu + H 2 O
3) Al(OH)3 + HCl→ AlCl 3 + H 2 O
4) HCl + Fe→ FeCl 2 + H 2
3 .Oxidant in the reaction scheme
CrO 3 + NH 3 → Cr 2 O 3 + H 2 O + N 2 is
Cr +6 2) N 3- 3) Cr +3 4) N 2 0
4. Which substance dissociates to form hydrogen ions?
1) sodium carbonate 2) sodium hydroxide
3) sulfuric acid 4) silicic acid
5. With complete dissociation, 1 mol of aluminum nitrate (III) in solution is formed
1) 3 mol of aluminum cations and 1 mol of nitrate ions
2) 2 mol of aluminum cations and 3 mol of nitrate ions
3) 1 mol of aluminum cations and 2 mol of nitrate ions
4) 1 mol of aluminum cations and 3 mol of nitrate ions
6. Choose the correct notation for the right side of the sodium phosphate dissociation equation
1) = Na + +PO 4 3- 2) = 3 Na + +PO 4 3-
3) = 2Na + +PO 4 3- 4) = Na + + HPO 4 2-
Part B
Establish a correspondence between the redox process, indicated by the electron transfer scheme, and the chemical reaction scheme.
1) S 0 →S 2- BUT . SO 2 + O 2 → SO 3
2) S 4+ →S 6+ B . NH 3 + O 2 →NO + H 2 O
3) N -3 → N +2 AT . S+O 2 →SO 2
4) N +4 →N +5 G . H 2 O + NO 2 + O 2 → HNO 3
D. H 2 + S → H 2 S
2. Set the correspondence between the reagents and the abbreviated ionic reaction equation
Reduced reagents ionic equations reactions
1) copper sulfate ( II) and sodium hydroxide A.CO 3 2- + 2 H + → H 2 O + CO 2
2) nitric acid and potassium hydroxide B.Cu 2+ + 2 Oh - → Cu(Oh) 2
3) potassium carbonate and hydrochloric acid B.CuO + 2 H + → Cu 2+ + H 2 O
4) copper oxide ( II) and hydrochloric acid D.H + + Oh - → H 2 O
3 . To shift the chemical equilibrium of reaction 2SO 2 + O 2 = 2 SO 3 + Q
towards the formation of sulfur oxide (VI) necessary
1) raise the temperature
2) lower the temperature
3) reduce pressure
4) increase pressure
5) reduce concentrationO 2
6) increase concentrationSO 2
Part C
Determine the amount of heat that will be released during the formation of 120 g of magnesium oxideas a result of the combustion reaction of magnesium, using the thermochemical equation.
2 mg + O 2 = 2 MgO+ 1204 kJ
The reaction, the equation of which is 2SO2 + O2 \u003d 2 SO3 + Q, is classified as a reaction:
a) irreversible, exothermic;
b) reversible, endothermic;
c) irreversible, endothermic;
d) reversible, exothermic
2. The temperature coefficient of the reaction is 2. Determine how many times the reaction rate will increase with an increase in temperature by 30 ° C.
3. Zinc and iron react with sulfuric acid of the same concentration. In which tube will the reaction rate be faster?
4. Some reaction, displayed by the equation A + 2 B \u003d AB2, proceeds in solution. The initial concentration of substance B was 4 mol/l. After 2 minutes, the concentration of substance B was 0.5 mol/L. Calculate the average rate of a chemical reaction.
5. Determine in which direction the equilibrium of the chemical reaction CH4 (g) + H2O (g) → CO2 (g) + H2 (g) - Q will shift when
a) an increase in temperature
b) pressure drop
c) an increase in the concentration of carbon dioxide.
6. The law of mass action: at a constant temperature, the rate of this reaction is proportional to the product ...
7. Systems in which there are no interfaces separating parts of the system from each other are called ...
8. How will the rate of a chemical reaction change, the equation of which
CO + 2H2 → CH3OH, with a 4-fold decrease in pressure in the reacting system?
9. How will the rate of a chemical reaction change, the equation of which
2NH3 + 3CuO = N2 + 3H2O + 3Cu if the ammonia concentration decreases by 3 times.
10. The temperature coefficient is 3. How many times will the rate of a chemical reaction increase with an increase in temperature by 30 ° C.
1. Make a classification scheme for chemical reactions: a) according to the number and composition of the starting materials and reaction products; b) by thermal2. Give examples of chemical reactions in which the qualitative composition of substances remains constant.
Please help, I need to solve a chemistry test 1) Chemical elements - magnesium, aluminum, sodium - arrange in order of strengthmetallic properties. For the element with the most pronounced metallic properties, draw a diagram of the structure of the atom. Compose a molecular formula. structural electronic formula of its compound.
2) Carry out the following transformations Fe->Fes->H2S->Na2S. Name all substances. Indicate the type of chemical reaction; for reaction 3, write the full ionic and abbreviated equations.
3) Complete the equations of those reactions that proceed to the end as a result of the formation of gas: a) CaCo3 + HCl ->... b) BaCl2 + AgNO3 ->... c) K2SO3 + HNO3 ->... d) Na2CO3 + KCl->...
4) Write the formulas of acids and their corresponding acid oxides carbonic, sulphurous, selenic.
5) (task) Mass nitric acid in 350 cm2 of a solution with a mass fraction of acid of 16% and completely 1.09 g / cm3, a solution of lithium hydroxide was added. Determine the mass and number of molecules of the formed salt. PLEASE HELP I would be very grateful