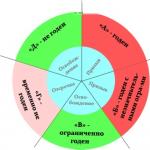
Let's move on to the consideration of task No. 5 in the OGE in chemistry or A5. This question is devoted to the classification of substances in chemistry, it discusses the main classes of inorganic substances and nomenclature. The question is quite capacious, so I made diagrams that will help to better understand.
Theory for task No. 5 OGE in chemistry
So, as we already discussed in the previous question A3, substances are simple and complex. Simple ones are made up of atoms of one element - complex ones are made up of atoms of different elements. Elements are further subdivided into metals and non-metals. Complex substances have more classes - oxides, acids, bases, alkalis.
Consider the classification of oxides. Oxides are compounds of oxygen with other elements. Depending on which element oxygen forms a compound with, oxides are divided into basic, acidic and amphoteric.
- Basic oxides form metals in oxidation states +1 and +2 (K2O, MgO)
- Acid oxides form predominantly non-metals (SO3, N2O5)
- Metals Zn and Al form amphoteric oxides (ZnO, Al2O3)
Of all the rules there are exceptions, but about them another time. In addition, these exceptions do not appear in the OGE and the Unified State Examination.

Classification of hydroxides
Hydroxides are products of the combination of oxides with water. Depending on what the oxide was, hydroxides are divided into bases, acids and amphoteric bases. Basic oxides form bases, acidic, respectively, acids, amphoteric oxides form amphoteric bases - substances that exhibit the properties of both acids and bases. In turn, the bases are divided into soluble - alkali, and insoluble.

Acids have different classifications. There are oxygen-containing and anoxic acids. The difference between the former and the latter is that the former contain oxygen in their molecule, while the latter consist only of an element and hydrogen (HCl, for example). Oxygen-free acids are formed directly by the interaction of an element (Cl2) and hydrogen (H2), while oxygen-containing acids are formed by the interaction of oxides with water.
Classification by basicity implies the number of protons given up by an acid molecule during complete dissociation. Monobasic acids dissociate to form one proton, dibasic acids to form two, and so on.
Classification according to the degree of dissociation shows how easy dissociation is (separation of a proton from an acid molecule). Depending on this, strong and weak acids are distinguished.
Salts are divided into medium, acidic and basic. Acid salts contain a proton, while basic salts contain a hydroxyl group. Acid salts are the product of the interaction of an excess of an acid with a base, basic salts, on the contrary, are the product of the interaction of an excess of a base with an acid.

Let's sum up a little on the topic.
- Oxides - complex substances made up of two chemical elements, one of which is oxygen .
- Grounds - metal ions and hydroxide ions .
- Acids - are complex substances hydrogen ions and acidic residues .
- Salts - are complex substances metal ions and acidic residues .
Analysis of typical options for task No. 5 OGE in chemistry
The first version of the assignment
Sodium hydroxide corresponds to the formula
- NaOH
- NaHCO3
- Na2CO3
Let's consider each case. NaH is a compound of sodium metal with hydrogen - such compounds are called hydrides , but not hydroxides. NaOH is formed by a metal cation - sodium and a hydroxo group. This is sodium hydroxide according to the classification. NaHCO3 - acid salt - sodium bicarbonate. It is formed by a carbonic acid residue and a sodium cation. Na 2 CO 3 - middle salt - sodium carbonate.
Tasks of the school Olympiad in chemistry
5 - 6 grade
Test
Choose one correct answer (1 point for each answer)
1. What gas is formed during photosynthesis:
2. Atom is...
3. Is a substance:
4. To separate the mixture, water - vegetable oil can be used to distinguish components by:
5. Chemical phenomena include:
Match: (2 points for each answer)
6.
1. simple2. complex
a) water
b) oxygen
c) nitrogen
d) carbon dioxide
e) sand
e) table salt
7.
1. pure substances2. mixtures
a) granite
b) oxygen
to the air
d) iron
e) hydrogen
f) soil
8.
1. chemical phenomena2. physical phenomena
a) iron rusting
b) metal melting
c) boiling water
d) burning food
e) leaf rot
e) dissolving sugar
9.
1. body2. substances
a) gold
b) coin
c) a chair
d) glass
e) vase
e) acetic acid
10. Distribute the ways of separating mixtures:
1. iron and sand2. water and salt
3. sand and water
a) the action of a magnet
b) filtering
c) evaporation
Tasks:
Walking through the forest in the summer, the student discovered on his way an anthill, in which a crow, spreading its wings, “took baths”, planting ants in feathers with its beak. Why did she do it? What chemical substance did the crow use while "bathing" in the anthill? (5 points)
The student decided to help his friend catch up on the missed material in chemistry, tell him about chemical phenomena: 1) heat comes from a radiator; 2) extinguishing soda with vinegar when preparing the dough; 3) melting butter in a frying pan; 4) adding sugar to tea; 5) juice fermentation; 6) sour milk; 7) the appearance of rust on the nails; 8) spreading the smell of perfume. Is the student right? Are all the processes listed by the student chemical? Are any of them physical? (5 points)
Cars, cars, literally everything is flooded ... What materials and substances are used to make modern cars. What phenomena (physical, chemical) are observed during the operation of the car? (7 points)
Why can't plastic birdhouses be made? (7 points)
You have been given a mixture of the following substances: iron, soot, table salt, copper. Propose a plan for the separation of these substances. What laboratory equipment would be required to separate this mixture? (7 points)
Answers to tests:
1 - b, c;2 - a, d, e, f
1 -b, d, e; 2- a, c, e
1 - a, d, e; 2 - b, c, f
1 – b, c, e; 2 - a, d, f
1-a;
2 - in;
3 - b
Answers to tasks:
2. The student is wrong. Among the listed phenomena there are also physical ones, namely: 1, 3, 4, 8.
3. Nowadays, in mechanical engineering, man-made materials are used, which are superior to metals in terms of lightness, strength, durability and other valuable properties. These are plastics, rubbers, rubber, glass, fiberglass and others. Thanks to them, modern machines can operate at high and low temperatures, deep underwater, in space. The chemical energy of the fuel (usually a liquid or gaseous hydrocarbon fuel) that burns in the working area is converted into mechanical work.
4. Plastic houses are extremely dangerous for birds, because plastics, unlike wood, are not able to absorb moisture and let it out through the smallest pores. Therefore, the water vapor released during breathing is absorbed by the litter and does not leave the house. High humidity is formed in the house, which is detrimental to birds.
5. Laboratory equipment: magnet, filter paper, funnel, beaker, spirit lamp.
1) we separate the iron with a magnet;
2) we dissolve the rest of the mixture in water, the salt dissolves, soot floats on top, copper sinks to the bottom;
3) filter the mixture - soot is filtered out, copper remains at the bottom of the glass;
4) there was a salt solution. Heat a thermal glass over an alcohol lamp - the water evaporates, the salt remains.
Gdz in chemistry grade 5
iasmmwas
Gdz in chemistry grade 5
GDZ in chemistry grade 5 - answers and a solution book.
The study of chemistry in the 5th grade is just beginning, but this process itself is very fast. Each topic takes only a few lessons, in addition, students need basic knowledge in related subjects - biology, physics, mathematics - so it is not surprising that help is often needed in solving tasks in chemistry. In this situation, the GDZ in chemistry grade 5 will help, which contain answers to questions for paragraphs of the textbook, theoretical tasks and workshops.
Chemistry is an interesting science, even the very process of its study is captivating, especially since chemists still continue to discover new elements, to obtain previously unknown reactions and substances. Difficulties that arise in the learning process are solved with the help of a teacher, a textbook and a solution book in chemistry grade 5, the ability to use Internet resources is convenient and accessible. By studying chemistry, it is easier to understand subjects related to it, especially botany and zoology, because all natural interactions are built on chemical reactions. Having answers in chemistry grade 5 online will help to significantly reduce the time spent on analysis and implementation homework. It must be remembered that at first you need to try to complete any task on your own: if you just write off "homework", then there will never be a positive result.
Will benefit GDz in chemistry for grade 5 and in the classroom, when doing workshops, various works during oral interviews. With their help, you can learn how to correctly formulate answers by looking at a sample in the collection, check in advance the correctness of the tasks.
Reshebnik in chemistry for grade 5
The best way to use the Grade 5 Chemistry Solutions Online is self-examination, that is, checking the task you completed yourself with the sample in the collection, analyzing the errors found and remembering the correct options. It was for this purpose that collections with ready-made homework assignments were originally prepared.
Also, the ability to get the correct answers in grade 8 chemistry will be useful to parents and teachers. The parents of any student may not remember the school material, and with all their desire, they are simply not able to help in solving household problems. The collection will tell you not only the correct answers, but also the progress of the tasks in chemistry of the 8th grade online, as a result, you can tell the student the direction of the solution. Teachers, using ready-made tasks, will facilitate and speed up their work on checking notebooks.
Online answers
If you have landed on this page, it means that you have problems with your homework in chemistry or you just want to check the correctness of your self-solved homework.
Both ready-made homework assignments in chemistry will help both of them. Select the required class and the author of the textbook for which the GDZ in chemistry is needed, and you will have the opportunity to download or watch online ready-made answers to the necessary tasks and examples.
Summer has passed, and we are again turning to chemistry. To take the next steps in studying it, let's review what you learned last year. Let's just list, and if this is not enough for you, if you see unfamiliar terms. Do not be lazy and refer to the textbook for the last class! Spending some not very much time on this now, you will save it much more later. Since it will be easier for you to perceive new material.
subject: Chemistry
So, the subject of chemistry is the interconversion of substances - chemical reactions.
Depending on the interacting substances and the conditions for conducting chemical reactions, they proceed at different rates. The rate of a reaction is the amount of a substance converted per unit of time per unit of reaction space. Mole / (l s) - for homogeneous reactions and mol / (m 2 s) - heterogeneous). This substance can be any participant in the reaction, both the reactant and the product. The rate of a reaction depends on the nature of the reactants - different substances interact at different rates. From temperature - the higher it is, the more intense the interaction. From concentration - the more substance in a unit volume, the more collisions between molecules. From catalysts substances that form intermediate compounds with reagents. What can sometimes happen at a faster rate than the direct interaction of the original substances.
During chemical reaction torn alone chemical bonds(energy is expended) and new bonds are formed (energy is released). Since the bond energies are different in different substances, as a result of a chemical reaction, energy is released (in an exothermic reaction) or energy is absorbed.
Ready-made homework in chemistry
Buying a GDZ in chemistry is the decision of many parents and teachers who seek to rationally use their time and at the same time take a responsible attitude to the student's studies. Reshebrnik is an essential attribute of effective learning. The Homework Book will provide the following benefits:
allows you to quickly check the task;
understand which rule is assimilated worse than the others, and work in this direction more intensively;
will give detailed explanations on all key elements of the tasks;
replace the consultation with the teacher, if it is difficult to conduct it in the near future;
saves time for completing assignments in other subjects.
Gdz in chemistry
GDZ in chemistry - will allow you to successfully master even the most complex material quickly and efficiently. Tasks that are difficult to complete at home will no longer be a problem for the family and the reason for going to bed late, and, therefore, inadequate rest.
And also the solver will provide invaluable assistance to the family of a frequently ill child. Even after a long absence from the classroom, the student will cope with any control work and will be able to successfully assimilate the subsequent material. GDZ in chemistry is a convenient way to study a topic at home. When being in a team is undesirable due to a state of health.
A collection of ready-made chemistry homework assignments is an opportunity to plan your day and pay due attention to other important things: sports, creativity, tutoring and just relaxing.
Today's school curriculum is oversaturated, each student has his own individual pace of learning, as well as abilities for a particular subject. These features are not taken into account by the developers of the school curriculum, which can have adverse consequences:
overwork;
loss of interest in learning;
deterioration in the perception of knowledge.
GDZ in chemistry and other complex subjects will help to avoid the above consequences, allowing the student to study with pleasure and fully learn the school curriculum.
The Gdz-vip website offers a wide selection of GDZ in chemistry, the Russian language, mathematics and other disciplines.
For the correct answer to each of the tasks 1-8, 12-16, 20, 21, 27-29, 1 point is given.
Tasks 9–11, 17–19, 22–26 are considered completed correctly if the sequence of numbers is correctly indicated. For a complete correct answer in tasks 9–11, 17–19, 22–26, 2 points are given; if one mistake is made - 1 point; for an incorrect answer (more than one mistake) or its absence - 0 points.
Theory on assignment:
| BUT | B | AT |
| 4 | 1 | 3 |
Non-salt-forming oxides include non-metal oxides with an oxidation state of +1, +2 (CO, NO, N 2 O, SiO), therefore, CO is a non-salt-forming oxide.
Mg(OH) 2 is a base- a complex substance consisting of a metal atom and one or more hydroxo groups (-OH). The general formula of the bases is: M (OH) y, where y is the number of hydroxo groups equal to the oxidation state of the metal M (usually +1 and +2). Bases are divided into soluble (alkali) and insoluble.
The products of the complete replacement of hydrogen atoms in an acid molecule by metal atoms or the complete replacement of hydroxo groups in a base molecule by acidic residues are called - medium salts- NH 4 NO 3 is a vivid example of this class of substances. 
Establish a correspondence between the formula of a substance and the class/group to which\(th) this substance belongs: for each position indicated by a letter, select the corresponding position indicated by a number.
| BUT | B | AT |
| 4 | 2 | 1 |
Let's write the formulas of substances:
Strontium oxide - SrO - will be basic oxide since it will react with acids.
 Types of oxides
Types of oxides  Oxides in the periodic table
Oxides in the periodic table Barium iodide - BaI 2 - medium salt, since all hydrogen atoms are replaced by a metal, and all hydroxy groups are replaced by acid residues.
Potassium dihydrogen phosphate - KH 2 PO 4 - acid salt, because hydrogen atoms in the acid are partially replaced by metal atoms. They are obtained by neutralizing a base with an excess of an acid. To properly name acid salt, it is necessary to add the prefix hydro- or dihydro- to the name of the normal salt, depending on the number of hydrogen atoms that make up the acid salt. For example, KHCO 3 is potassium bicarbonate, KH 2 PO 4 is potassium dihydroorthophosphate. It must be remembered that acid salts can only form two or more basic acids.
Establish a correspondence between the formula of a substance and the class/group to which\(th) this substance belongs: for each position indicated by a letter, select the corresponding position indicated by a number.
| BUT | B | AT |
| 1 | 3 | 1 |
SO 3 and P 2 O 3 are acidic oxides, as they react with bases and are oxides of non-metals with an oxidation state >+5.
Na 2 O is a typical basic oxide, because it is a metal oxide with an oxidation state of +1. It reacts with acids.
Establish a correspondence between the formula of a substance and the class/group to which\(th) this substance belongs: for each position indicated by a letter, select the corresponding position indicated by a number.
| BUT | B | AT |
| 4 | 1 | 2 |
Fe 2 O 3 - amphoteric oxide, since it reacts with both bases and acids, in addition, it is a metal oxide with an oxidation state of +3, which also indicates its amphoterism.
Na 2 - complex salt, anion 2- is presented instead of the acid residue.
HNO 3 - acid- (acid hydroxides) is a complex substance consisting of hydrogen atoms that can be replaced by metal atoms, and acid residues. The general formula of acids: H x Ac, where Ac is an acid residue (from the English "acid" - acid), x is the number of hydrogen atoms equal to the charge of the ion of the acid residue.
Methodology for solving problems in chemistry
When solving problems, you need to be guided by a few simple rules:
- Carefully read the condition of the problem;
- Write down what is given;
- Convert, if necessary, units physical quantities to SI units (some non-SI units are allowed, such as liters);
- Write down, if necessary, the reaction equation and arrange the coefficients;
- Solve the problem using the concept of the amount of substance, and not the method of drawing up proportions;
- Write down the answer.
In order to successful preparation in chemistry, you should carefully consider the solutions to the problems given in the text, as well as independently solve a sufficient number of them. It is in the process of solving problems that the main theoretical provisions of the chemistry course will be fixed. It is necessary to solve problems throughout the entire time of studying chemistry and preparing for the exam.
You can use the tasks on this page, or you can download a good collection of tasks and exercises with the solution of typical and complicated tasks (M. I. Lebedeva, I. A. Ankudimova): download.
Mole, molar mass
Molar mass is the ratio of the mass of a substance to the amount of a substance, i.e.
М(х) = m(x)/ν(x), (1)
where M(x) is the molar mass of substance X, m(x) is the mass of substance X, ν(x) is the amount of substance X. The SI unit for molar mass is kg/mol, but g/mol is commonly used. The unit of mass is g, kg. The SI unit for the amount of a substance is the mole.
Any chemistry problem solved through the amount of matter. Remember the basic formula:
ν(x) = m(x)/ М(х) = V(x)/V m = N/N A , (2)
where V(x) is the volume of substance Х(l), Vm is the molar volume of gas (l/mol), N is the number of particles, N A is the Avogadro constant.
1. Determine the mass sodium iodide NaI amount of substance 0.6 mol.
Given: ν(NaI)= 0.6 mol.
Find: m(NaI) =?
Solution. The molar mass of sodium iodide is:
M(NaI) = M(Na) + M(I) = 23 + 127 = 150 g/mol
Determine the mass of NaI:
m(NaI) = ν(NaI) M(NaI) = 0.6 150 = 90 g.
2. Determine the amount of substance atomic boron contained in sodium tetraborate Na 2 B 4 O 7 weighing 40.4 g.
Given: m(Na 2 B 4 O 7) \u003d 40.4 g.
Find: ν(B)=?
Solution. The molar mass of sodium tetraborate is 202 g/mol. Determine the amount of substance Na 2 B 4 O 7:
ν (Na 2 B 4 O 7) \u003d m (Na 2 B 4 O 7) / M (Na 2 B 4 O 7) \u003d 40.4 / 202 \u003d 0.2 mol.
Recall that 1 mol of sodium tetraborate molecule contains 2 mol of sodium atoms, 4 mol of boron atoms and 7 mol of oxygen atoms (see the formula of sodium tetraborate). Then the amount of atomic boron substance is: ν (B) \u003d 4 ν (Na 2 B 4 O 7) \u003d 4 0.2 \u003d 0.8 mol.
Calculations by chemical formulas. Mass share.
The mass fraction of a substance is the ratio of the mass of a given substance in the system to the mass of the entire system, i.e. ω(X) =m(X)/m, where ω(X) is the mass fraction of substance X, m(X) is the mass of substance X, m is the mass of the entire system. Mass fraction is a dimensionless quantity. It is expressed as a fraction of a unit or as a percentage. For example, the mass fraction of atomic oxygen is 0.42, or 42%, i.e. ω(O)=0.42. The mass fraction of atomic chlorine in sodium chloride is 0.607, or 60.7%, i.e. ω(Cl)=0.607.
3. Determine the mass fraction water of crystallization in barium chloride dihydrate BaCl 2 2H 2 O.
Solution: The molar mass of BaCl 2 2H 2 O is:
M (BaCl 2 2H 2 O) \u003d 137+ 2 35.5 + 2 18 \u003d 244 g / mol
From the formula BaCl 2 2H 2 O it follows that 1 mol of barium chloride dihydrate contains 2 mol of H 2 O. From this we can determine the mass of water contained in BaCl 2 2H 2 O:
m(H 2 O) \u003d 2 18 \u003d 36 g.
We find the mass fraction of water of crystallization in barium chloride dihydrate BaCl 2 2H 2 O.
ω (H 2 O) \u003d m (H 2 O) / m (BaCl 2 2H 2 O) \u003d 36/244 \u003d 0.1475 \u003d 14.75%.
4. From sample rock weighing 25 g, containing the mineral argentite Ag 2 S, silver was isolated weighing 5.4 g. Determine the mass fraction argentite in the sample.
Given: m(Ag)=5.4 g; m = 25 g.
Find: ω(Ag 2 S) =?
Solution: we determine the amount of silver substance in argentite: ν (Ag) \u003d m (Ag) / M (Ag) \u003d 5.4 / 108 \u003d 0.05 mol.
From the formula Ag 2 S it follows that the amount of argentite substance is half the amount of silver substance. Determine the amount of argentite substance:
ν (Ag 2 S) \u003d 0.5 ν (Ag) \u003d 0.5 0.05 \u003d 0.025 mol
We calculate the mass of argentite:
m (Ag 2 S) \u003d ν (Ag 2 S) M (Ag 2 S) \u003d 0.025 248 \u003d 6.2 g.
Now we determine the mass fraction of argentite in a rock sample, weighing 25 g.
ω (Ag 2 S) \u003d m (Ag 2 S) / m \u003d 6.2 / 25 \u003d 0.248 \u003d 24.8%.
Derivation of compound formulas
5. Determine the simplest compound formula potassium with manganese and oxygen, if the mass fractions of elements in this substance are 24.7, 34.8 and 40.5%, respectively.
Given: ω(K)=24.7%; ω(Mn)=34.8%; ω(O)=40.5%.
Find: compound formula.
Solution: for calculations, we select the mass of the compound, equal to 100 g, i.e. m=100 g. Masses of potassium, manganese and oxygen will be:
m (K) = m ω (K); m (K) \u003d 100 0.247 \u003d 24.7 g;
m (Mn) = m ω(Mn); m (Mn) = 100 0.348 = 34.8 g;
m (O) = m ω(O); m (O) \u003d 100 0.405 \u003d 40.5 g.
We determine the amount of substances of atomic potassium, manganese and oxygen:
ν (K) \u003d m (K) / M (K) \u003d 24.7 / 39 \u003d 0.63 mol
ν (Mn) \u003d m (Mn) / M (Mn) \u003d 34.8 / 55 \u003d 0.63 mol
ν (O) \u003d m (O) / M (O) \u003d 40.5 / 16 \u003d 2.5 mol
We find the ratio of the amounts of substances:
ν(K) : ν(Mn) : ν(O) = 0.63: 0.63: 2.5.
Dividing right side equality to a smaller number (0.63) we get:
ν(K) : ν(Mn) : ν(O) = 1: 1: 4.
Therefore, the simplest formula of the KMnO 4 compound.
6. During the combustion of 1.3 g of the substance, 4.4 g of carbon monoxide (IV) and 0.9 g of water were formed. Find the molecular formula substance if its hydrogen density is 39.
Given: m(in-va) \u003d 1.3 g; m(CO 2)=4.4 g; m(H 2 O)=0.9 g; D H2 \u003d 39.
Find: the formula of the substance.
Solution: Assume that the substance you are looking for contains carbon, hydrogen and oxygen, because during its combustion, CO 2 and H 2 O were formed. Then it is necessary to find the amounts of substances CO 2 and H 2 O in order to determine the amounts of substances of atomic carbon, hydrogen and oxygen.
ν (CO 2) \u003d m (CO 2) / M (CO 2) \u003d 4.4 / 44 \u003d 0.1 mol;
ν (H 2 O) \u003d m (H 2 O) / M (H 2 O) \u003d 0.9 / 18 \u003d 0.05 mol.
We determine the amount of substances of atomic carbon and hydrogen:
ν(C)= ν(CO 2); v(C)=0.1 mol;
ν(H)= 2 ν(H 2 O); ν (H) \u003d 2 0.05 \u003d 0.1 mol.
Therefore, the masses of carbon and hydrogen will be equal:
m(C) = ν(C) M(C) = 0.1 12 = 1.2 g;
m (H) \u003d ν (H) M (H) \u003d 0.1 1 \u003d 0.1 g.
We determine the qualitative composition of the substance:
m (in-va) \u003d m (C) + m (H) \u003d 1.2 + 0.1 \u003d 1.3 g.
Consequently, the substance consists only of carbon and hydrogen (see the condition of the problem). Let us now determine its molecular weight, based on the given in the condition tasks density of a substance with respect to hydrogen.
M (in-va) \u003d 2 D H2 \u003d 2 39 \u003d 78 g / mol.
ν(C) : ν(H) = 0.1: 0.1
Dividing the right side of the equation by the number 0.1, we get:
ν(C) : ν(H) = 1: 1
Let's take the number of carbon (or hydrogen) atoms as "x", then, multiplying "x" by atomic masses carbon and hydrogen and equating this amount to the molecular weight of the substance, we solve the equation:
12x + x \u003d 78. Hence x \u003d 6. Therefore, the formula of the substance C 6 H 6 is benzene.
Molar volume of gases. Laws of ideal gases. Volume fraction.
The molar volume of a gas is equal to the ratio of the volume of gas to the amount of substance of this gas, i.e.
Vm = V(X)/ ν(x),
where V m is the molar volume of gas - constant for any gas under given conditions; V(X) is the volume of gas X; ν(x) - the amount of gas substance X. The molar volume of gases under normal conditions (normal pressure p n \u003d 101 325 Pa ≈ 101.3 kPa and temperature Tn \u003d 273.15 K ≈ 273 K) is V m \u003d 22.4 l /mol.
In calculations involving gases, it is often necessary to switch from these conditions to normal conditions or vice versa. In this case, it is convenient to use the formula following from the combined gas law of Boyle-Mariotte and Gay-Lussac:
──── = ─── (3)
Where p is pressure; V is the volume; T is the temperature in the Kelvin scale; the index "n" indicates normal conditions.
The composition of gas mixtures is often expressed using a volume fraction - the ratio of the volume of a given component to the total volume of the system, i.e.
where φ(X) is the volume fraction of the X component; V(X) is the volume of the X component; V is the volume of the system. The volume fraction is a dimensionless quantity, it is expressed in fractions of a unit or as a percentage.
7. What volume takes at a temperature of 20 ° C and a pressure of 250 kPa ammonia weighing 51 g?
Given: m(NH 3)=51 g; p=250 kPa; t=20°C.
Find: V(NH 3) \u003d?
Solution: determine the amount of ammonia substance:
ν (NH 3) \u003d m (NH 3) / M (NH 3) \u003d 51/17 \u003d 3 mol.
The volume of ammonia under normal conditions is:
V (NH 3) \u003d V m ν (NH 3) \u003d 22.4 3 \u003d 67.2 l.
Using formula (3), we bring the volume of ammonia to these conditions [temperature T \u003d (273 + 20) K \u003d 293 K]:
p n TV n (NH 3) 101.3 293 67.2
V (NH 3) \u003d ──────── \u003d ────────── \u003d 29.2 l.
8. Determine volume, which will take under normal conditions a gas mixture containing hydrogen, weighing 1.4 g and nitrogen, weighing 5.6 g.
Given: m(N 2)=5.6 g; m(H2)=1.4; well.
Find: V(mixture)=?
Solution: find the amount of substance hydrogen and nitrogen:
ν (N 2) \u003d m (N 2) / M (N 2) \u003d 5.6 / 28 \u003d 0.2 mol
ν (H 2) \u003d m (H 2) / M (H 2) \u003d 1.4 / 2 \u003d 0.7 mol
Since under normal conditions these gases do not interact with each other, the volume of the gas mixture will be equal to the sum of the volumes of gases, i.e.
V (mixtures) \u003d V (N 2) + V (H 2) \u003d V m ν (N 2) + V m ν (H 2) \u003d 22.4 0.2 + 22.4 0.7 \u003d 20.16 l.
Calculations by chemical equations
Calculations according to chemical equations (stoichiometric calculations) are based on the law of conservation of the mass of substances. However, in real chemical processes, due to an incomplete reaction and various losses of substances, the mass of the resulting products is often less than that which should be formed in accordance with the law of conservation of the mass of substances. The yield of the reaction product (or the mass fraction of the yield) is the ratio of the mass of the actually obtained product, expressed as a percentage, to its mass, which should be formed in accordance with the theoretical calculation, i.e.
η = /m(X) (4)
Where η is the product yield, %; m p (X) - the mass of the product X obtained in the real process; m(X) is the calculated mass of substance X.
In those tasks where the product yield is not specified, it is assumed that it is quantitative (theoretical), i.e. η=100%.
9. What mass of phosphorus should be burned for getting phosphorus oxide (V) weighing 7.1 g?
Given: m(P 2 O 5) \u003d 7.1 g.
Find: m(P) =?
Solution: we write the equation for the combustion reaction of phosphorus and arrange the stoichiometric coefficients.
4P+ 5O 2 = 2P 2 O 5
We determine the amount of substance P 2 O 5 obtained in the reaction.
ν (P 2 O 5) \u003d m (P 2 O 5) / M (P 2 O 5) \u003d 7.1 / 142 \u003d 0.05 mol.
It follows from the reaction equation that ν (P 2 O 5) \u003d 2 ν (P), therefore, the amount of phosphorus substance required in the reaction is:
ν (P 2 O 5) \u003d 2 ν (P) \u003d 2 0.05 \u003d 0.1 mol.
From here we find the mass of phosphorus:
m(Р) = ν(Р) М(Р) = 0.1 31 = 3.1 g.
10. Magnesium weighing 6 g and zinc weighing 6.5 g were dissolved in an excess of hydrochloric acid. What volume hydrogen, measured under normal conditions, stand out wherein?
Given: m(Mg)=6 g; m(Zn)=6.5 g; well.
Find: V(H 2) =?
Solution: we write down the reaction equations for the interaction of magnesium and zinc with hydrochloric acid and arrange the stoichiometric coefficients.
Zn + 2 HCl \u003d ZnCl 2 + H 2
Mg + 2 HCl \u003d MgCl 2 + H 2
We determine the amount of magnesium and zinc substances that reacted with hydrochloric acid.
ν(Mg) \u003d m (Mg) / M (Mg) \u003d 6/24 \u003d 0.25 mol
ν (Zn) \u003d m (Zn) / M (Zn) \u003d 6.5 / 65 \u003d 0.1 mol.
It follows from the reaction equations that the amount of the substance of the metal and hydrogen are equal, i.e. ν (Mg) \u003d ν (H 2); ν (Zn) \u003d ν (H 2), we determine the amount of hydrogen resulting from two reactions:
ν (Н 2) \u003d ν (Mg) + ν (Zn) \u003d 0.25 + 0.1 \u003d 0.35 mol.
We calculate the volume of hydrogen released as a result of the reaction:
V (H 2) \u003d V m ν (H 2) \u003d 22.4 0.35 \u003d 7.84 l.
11. When passing hydrogen sulfide with a volume of 2.8 liters (normal conditions) through an excess of copper (II) sulfate solution, a precipitate was formed weighing 11.4 g. Determine the exit reaction product.
Given: V(H 2 S)=2.8 l; m(precipitate)= 11.4 g; well.
Find: η =?
Solution: we write the reaction equation for the interaction of hydrogen sulfide and copper (II) sulfate.
H 2 S + CuSO 4 \u003d CuS ↓ + H 2 SO 4
Determine the amount of hydrogen sulfide substance involved in the reaction.
ν (H 2 S) \u003d V (H 2 S) / V m \u003d 2.8 / 22.4 \u003d 0.125 mol.
It follows from the reaction equation that ν (H 2 S) \u003d ν (СuS) \u003d 0.125 mol. So you can find the theoretical mass of CuS.
m(CuS) \u003d ν (CuS) M (CuS) \u003d 0.125 96 \u003d 12 g.
Now we determine the product yield using formula (4):
η = /m(X)= 11.4 100/ 12 = 95%.
12. What weight ammonium chloride is formed by the interaction of hydrogen chloride weighing 7.3 g with ammonia weighing 5.1 g? What gas will be left in excess? Determine the mass of the excess.
Given: m(HCl)=7.3 g; m(NH 3) \u003d 5.1 g.
Find: m(NH 4 Cl) =? m(excess) =?
Solution: write the reaction equation.
HCl + NH 3 \u003d NH 4 Cl
This task is for "excess" and "deficiency". We calculate the amount of hydrogen chloride and ammonia and determine which gas is in excess.
ν(HCl) \u003d m (HCl) / M (HCl) \u003d 7.3 / 36.5 \u003d 0.2 mol;
ν (NH 3) \u003d m (NH 3) / M (NH 3) \u003d 5.1 / 17 \u003d 0.3 mol.
Ammonia is in excess, so the calculation is based on the deficiency, i.e. by hydrogen chloride. It follows from the reaction equation that ν (HCl) \u003d ν (NH 4 Cl) \u003d 0.2 mol. Determine the mass of ammonium chloride.
m (NH 4 Cl) \u003d ν (NH 4 Cl) M (NH 4 Cl) \u003d 0.2 53.5 \u003d 10.7 g.
We determined that ammonia is in excess (according to the amount of substance, the excess is 0.1 mol). Calculate the mass of excess ammonia.
m (NH 3) \u003d ν (NH 3) M (NH 3) \u003d 0.1 17 \u003d 1.7 g.
13. Technical calcium carbide weighing 20 g was treated with excess water, obtaining acetylene, passing through which through an excess of bromine water formed 1,1,2,2-tetrabromoethane weighing 86.5 g. Determine mass fraction SaS 2 in technical carbide.
Given: m = 20 g; m(C 2 H 2 Br 4) \u003d 86.5 g.
Find: ω (CaC 2) =?
Solution: we write down the equations of interaction of calcium carbide with water and acetylene with bromine water and arrange the stoichiometric coefficients.
CaC 2 +2 H 2 O \u003d Ca (OH) 2 + C 2 H 2
C 2 H 2 +2 Br 2 \u003d C 2 H 2 Br 4
Find the amount of substance tetrabromoethane.
ν (C 2 H 2 Br 4) \u003d m (C 2 H 2 Br 4) / M (C 2 H 2 Br 4) \u003d 86.5 / 346 \u003d 0.25 mol.
It follows from the reaction equations that ν (C 2 H 2 Br 4) \u003d ν (C 2 H 2) \u003d ν (CaC 2) \u003d 0.25 mol. From here we can find the mass of pure calcium carbide (without impurities).
m (CaC 2) \u003d ν (CaC 2) M (CaC 2) \u003d 0.25 64 \u003d 16 g.
We determine the mass fraction of CaC 2 in technical carbide.
ω (CaC 2) \u003d m (CaC 2) / m \u003d 16/20 \u003d 0.8 \u003d 80%.
Solutions. Mass fraction of the solution component
14. Sulfur weighing 1.8 g was dissolved in benzene with a volume of 170 ml. The density of benzene is 0.88 g / ml. Determine mass fraction sulfur in solution.
Given: V(C 6 H 6) =170 ml; m(S) = 1.8 g; ρ(C 6 C 6)=0.88 g/ml.
Find: ω(S) =?
Solution: to find the mass fraction of sulfur in the solution, it is necessary to calculate the mass of the solution. Determine the mass of benzene.
m (C 6 C 6) \u003d ρ (C 6 C 6) V (C 6 H 6) \u003d 0.88 170 \u003d 149.6 g.
Find the total mass of the solution.
m (solution) \u003d m (C 6 C 6) + m (S) \u003d 149.6 + 1.8 \u003d 151.4 g.
Calculate the mass fraction of sulfur.
ω(S) =m(S)/m=1.8 /151.4 = 0.0119 = 1.19%.
15. Iron sulfate FeSO 4 7H 2 O weighing 3.5 g was dissolved in water weighing 40 g. Determine mass fraction of iron sulfate (II) in the resulting solution.
Given: m(H 2 O)=40 g; m (FeSO 4 7H 2 O) \u003d 3.5 g.
Find: ω(FeSO 4) =?
Solution: find the mass of FeSO 4 contained in FeSO 4 7H 2 O. To do this, calculate the amount of substance FeSO 4 7H 2 O.
ν (FeSO 4 7H 2 O) \u003d m (FeSO 4 7H 2 O) / M (FeSO 4 7H 2 O) \u003d 3.5 / 278 \u003d 0.0125 mol
From the formula of ferrous sulfate it follows that ν (FeSO 4) \u003d ν (FeSO 4 7H 2 O) \u003d 0.0125 mol. Calculate the mass of FeSO 4:
m (FeSO 4) \u003d ν (FeSO 4) M (FeSO 4) \u003d 0.0125 152 \u003d 1.91 g.
Considering that the mass of the solution consists of the mass of ferrous sulfate (3.5 g) and the mass of water (40 g), we calculate the mass fraction of ferrous sulfate in the solution.
ω (FeSO 4) \u003d m (FeSO 4) / m \u003d 1.91 / 43.5 \u003d 0.044 \u003d 4.4%.
Tasks for independent solution
- 50 g of methyl iodide in hexane were treated with sodium metal, and 1.12 liters of gas, measured under normal conditions, were released. Determine the mass fraction of methyl iodide in the solution. Answer: 28,4%.
- Some alcohol was oxidized to form a monobasic carboxylic acid. When burning 13.2 g of this acid, carbon dioxide was obtained, for the complete neutralization of which it took 192 ml of a KOH solution with a mass fraction of 28%. The density of the KOH solution is 1.25 g/ml. Determine the formula for alcohol. Answer: butanol.
- Gas obtained by the interaction of 9.52 g of copper with 50 ml of an 81% solution nitric acid, with a density of 1.45 g/ml, was passed through 150 ml of a 20% NaOH solution with a density of 1.22 g/ml. Determine the mass fractions of dissolved substances. Answer: 12.5% NaOH; 6.48% NaNO 3 ; 5.26% NaNO 2 .
- Determine the volume of gases released during the explosion of 10 g of nitroglycerin. Answer: 7.15 l.
- Sample organic matter weighing 4.3 g was burned in oxygen. The reaction products are carbon monoxide (IV) with a volume of 6.72 liters (normal conditions) and water with a mass of 6.3 g. The vapor density of the starting substance for hydrogen is 43. Determine the formula of the substance. Answer: C 6 H 14 .